How Gases are Transported In The Body
Published (updated: ).
Matter is typically conveyed in 3 states. The state that one finds the matter depends on air pressure (or lack thereof, say, in a vacuum) and temperature. Iron, a solid at room temperature, becomes a liquid at 2800 degrees Fahrenheit (really, REALLY hot) and a gas at 5182 degrees Fahrenheit (about half the temperature of the sun). When things are hot, the molecules and atoms move around more and faster, and when they are cold they are slower
| gas | liquid | solid |
| assumes the shape and volume of its container particles can move past one another | assumes the shape of the part of the container which it occupies particles can move/slide past one another | retains a fixed volume and shape rigid – particles locked into place |
| compressible lots of free space between particles | not easily compressible little free space between particles | not easily compressible little free space between particles |
| flows easily particles can move past one another | flows easily particles can move/slide past one another | does not flow easily rigid – particles cannot move/slide past one another |

Gases dissolve in liquids, but usually only to a small extent. When a gas dissolves in a liquid, the ability of the gas molecules to move freely throughout the volume of the solvent is greatly restricted. If this latter volume is small, as is often the case, the gas is effectively being compressed. Both of these effects amount to a decrease in the entropy of the gas that is not usually compensated by the entropy increase due to mixing of the two kinds of molecules. Such processes greatly restrict the solubility of gases in liquids.

Oxygen and Carbon Dioxide (CO2) Are Carried Through The Body Dissolved In Plasma
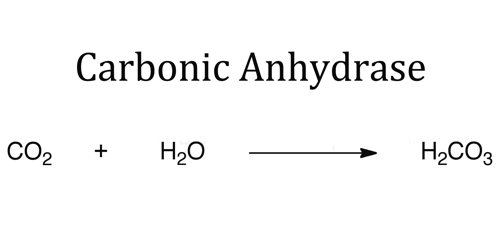
Carbon dioxide is 20 times more soluble (in liquids) than oxygen; it obeys Henry’s law, which states that the number of molecules in solution is proportional to the partial pressure at the liquid surface. Arterial blood will contain about 2.5 ml of carbon dioxide per 100 ml of venous. Another method of transporting carbon dioxide is binding the carbon dioxide with water to create carbonic acid. The enzyme carbonic anhydrase is present in a number of organs of the body including the eye, kidney and brain. Once carbonic acid is formed it dissociates easily so that the ratio of H2CO3 (carbonic acid) to HCO3 (bicarbonate) is 1:20.
Oxygen and Carbon Dioxide (CO2) Are Also Carried Through The Body Attached To Hemoglobin
Hemoglobin is a protein molecule found in red blood cells (erythrocytes) made of four subunits: two alpha subunits and two beta subunits. Each subunit surrounds a central heme group that contains iron and binds one oxygen molecule, allowing each hemoglobin molecule to bind four oxygen molecules. Molecules with more oxygen bound to the heme groups are brighter red. As a result, oxygenated arterial blood where the hemoglobin is carrying four oxygen molecules is bright red, while venous blood that is deoxygenated is darker red. When carbon dioxide binds to hemoglobin, a molecule called carbaminohemoglobin is formed. Binding of carbon dioxide to hemoglobin is reversible. Therefore, when it reaches the lungs, the carbon dioxide can freely dissociate from the hemoglobin and be expelled from the body.
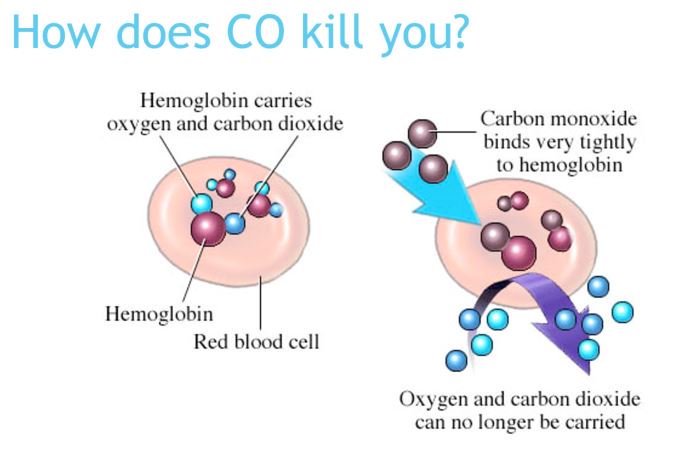
While carbon dioxide can readily associate and dissociate from hemoglobin, other molecules such as carbon monoxide (CO) cannot. Carbon monoxide has a greater affinity for hemoglobin than oxygen. Therefore, when carbon monoxide is present, it binds to hemoglobin preferentially over oxygen. As a result, oxygen cannot bind to hemoglobin, so very little oxygen is transported through the body. Carbon monoxide is a colorless, odorless gas and is therefore difficult to detect. It is produced by gas-powered vehicles and tools. Carbon monoxide can cause headaches, confusion, and nausea; long-term exposure can cause brain damage or death. Administering 100 percent (pure) oxygen is the usual treatment for carbon monoxide poisoning. Administration of pure oxygen speeds up the separation of carbon monoxide from hemoglobin.
