The Dead Space Gets Filled Up Before Any Air Gets Into The Lungs
Published (updated: ).
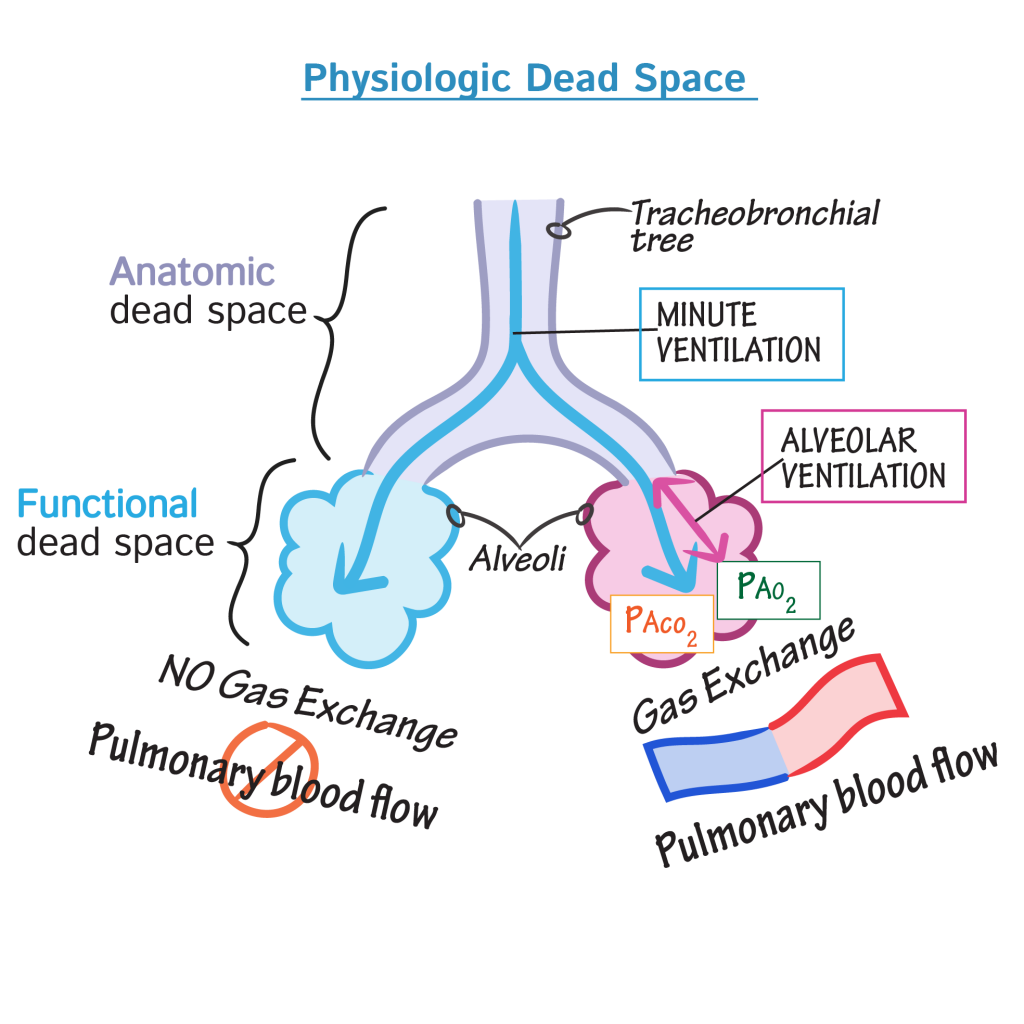
Dead space represents the volume of ventilated air that does not participate in gas exchange. The two types of dead space are anatomical dead space and physiologic dead space. Anatomical dead space is represented by the volume of air that fills the conducting zone of respiration made up by the nose, trachea, and bronchi. This volume is considered to be 30% of normal tidal volume (500 mL); therefore, the value of anatomic dead space is 150 mL. Physiologic or total dead space is equal to anatomic plus alveolar dead space which is the volume of air in the respiratory zone that does not take part in gas exchange. The respiratory zone is comprised of respiratory bronchioles, alveolar duct, alveolar sac, and alveoli. In a healthy adult alveolar dead space can be considered negligible. Therefore, physiologic dead space is equivalent to anatomical. One can see an increase in the value of physiologic dead space in lung disease states where the diffusion membrane of alveoli does not function properly or when there are ventilation/perfusion mismatch defects.
Ventilation is the manner by which air enters the lungs. There are two equations needed to calculate the volume that enters the lungs and the volume that reaches the alveoli. The volume that enters the lung per minute is known as minute ventilation (VE). The equation states VE equals tidal volume (VT) multiplied by respiratory rate (RR). This equation demonstrates that the total volume entering the lung is not equivalent to the total volume of gas reaching the alveoli because it does not factor in the gas in the anatomical dead space resting in the conductive airway. Thus, to know the volume of gas that reaches the alveoli per unit time we use the alveolar ventilation equation which states; alveolar ventilation (VA) equals VT minus physiologic dead space (VD) multiplied by RR. From this equation, clinicians can determine that the total volume gas inspired is not being fully utilized in the gas exchange due to the constant anatomical dead space.
Until now, clinicians have assumed the patient is a healthy individual with properly functioning alveoli. In disease states where alveoli have lost function, there will be a decrease in gas exchange and an increase in alveolar dead space. This can be seen most rapidly with sudden decreases in perfused to ventilated alveoli. This is usually seen in an abrupt decrease in cardiac output, hypotension or pulmonary embolism, due to fat, air, or amniotic fluid. While obstruction can cause decreased perfusion in PE, the greatest decrease in pulmonary blood flow is due to vasoconstriction caused by locally released vasoactive substances. In these situations, a lack of gas exchange at the alveolar level results in a decrease of PaCO2 gas being exchange by the remaining healthy alveoli and ultimately a lower PeCO2.
Clinically, disease states and environmental factors, such as smoking, all play a major role in the increase of dead space. Increases in dead space can be seen in lung disease states including emphysema, pneumonia, and acute respiratory distress syndrome (ARDS). Emphysema results in enlargement of air spaces and decreases in the diffusing capacity of the alveolar membrane due to the destruction of alveolar walls. In ARDS there is endothelial damage leading to an increase in the alveolar capillary permeability, thereby leading to leakage protein-rich fluid into the alveolar. This results in the formation of intra-alveolar hyaline membranes, which decrease the exchange of CO2 and O2 in the lung contributing to a larger dead space. Studies looking at the causes of death in this disease have shown an increase in dead space in the non-survivors versus survivors. The strongest association of an increased volume of dead space with mortality risk is seen in patients with ARDS.
Clinicians use the understanding of dead space to manage mechanically ventilated patients. Even in a healthy patient, ventilation via an endotracheal tube will increase the dead space volume because the breathing circuit does not participate in gas exchange. During mechanical ventilation, capnography is used; this records the volume of expired CO2, a value used to determine the physiologic dead space in patients. Adjustments in ventilation rates and the use of positive end-expiratory pressure (PEEP) are used to decrease dead space. Although multiple studies have failed to show this expected effect consistently, it is still widely used in cases of ARDS. Proper use of mechanical ventilation, as well as PEEP, is important considering both have been implicated in causing lung injury. Also, the use of high flow nasal cannula has been shown to decrease dead space in patients with acute and chronic respiratory diseases. This is largely due to decreasing rebreathing in the existing anatomic dead space. Understanding dead space and being able to calculate it is a vital tool for physicians dealing with ventilated, critically ill patients.
