The Effects of Acidosis on Cells & Tissue
Published .
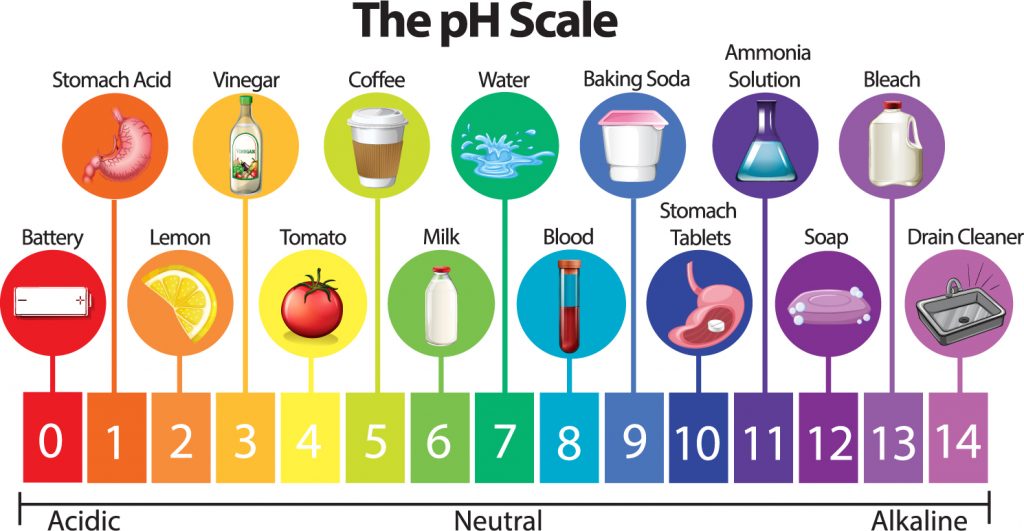
The measurement of pH, which is short for the potential of hydrogen ion concentration, is an important concept in chemistry that measures the acidity level of a solution. Since biological systems need a healthy balance between factors in which to operate, any changes to the pH level can disrupt living systems.
pH measures the potential of hydrogen in a given compound. Hydrogen is the building block of acids. Hydrogen can bond with nearly anything to create an acid through the process of covalent bonding. The lower the pH, the more hydrogen is present in the compound being studied.
The pH of a subject is rated on a 14-point scale. Pure water has a neutral pH close to 7.0 at 77 degrees Fahrenheit. Solutions less than this are acidic, while anything greater are base. Each subsequent number represents a tenfold difference over the previous one.
Acid-Base Homeostasis
Acid-base homeostasis is the function by which normal pH levels are maintained in an organism. Many important buffer agents act to regulate imbalances. In the bicarbonate buffering system, for instance, carbon dioxide can be combined with water to form carbonic acid, which dissociates to form a hydrogen ion and bicarbonate. The reverse reaction can happen if it is catalyzed by an enzyme. This may increase acidity or base levels according to need. In order to maintain the amount of carbon dioxide in circulation, respiratory functions change so that a balance can be reached.
pH Levels in Blood
Blood must stay within the careful range of 7.35 to 7.45. Excess acid within the blood is known as acidosis, and excess base is known as alkalosis. Any deviation on blood pH levels can alter the charge that keeps red blood cells apart and change the function or health of other organs and systems within the body. Since bones are often used as a mineral source for pH buffering, for instance, they are sensitive to changes in blood pH levels. Deviations may alter bone density.
Gastric Acid
A very common use of acid in an organism is gastric acid in the stomach, which consists mostly of hydrochloric acid combined with potassium chloride and sodium chloride. Its pH level is 1 to 2. When digesting food enters the stomach, acids begin to break down a protein structure and then its bonds. Antacid tablets can neutralize excess stomach acid if it is causing discomfort.
Acidosis Can Affect Perfusion
A slight change in pH in the spinal fluid and cerebral fluid during acidosis causes a reduction in affinity of hemoglobin for oxygen, reducing the critical oxygen supply to brain cells. Acute acidosis leads to lethargy and mental confusion. During alkalosis, or a rise in pH, blood vessels constrict and thereby reduce the supply of blood and oxygen to brain cells. Alkalosis can result in confusion, seizures and loss of consciousness.
Immune Cells
When the pH of blood declines below 7.35 during acidosis, immune cells, such as macrophages, release inflammatory cytokines, which cause inflammation. Acidosis also impairs the response of lymphocytes to fight pathogens, resulting in a poor immune response.
Bone Cells
Acidosis has an adverse effect on bone, causing an increase in loss of calcium. When blood pH drops below pH 7.35, osteoclast cells become activated and resorb, or destroy, bone. In bone cell experiments, a drop of pH of less than 0.1 doubled the amount of bone resorbed by osteoclasts. During normal bone remodeling, osteoclasts resorb bone and osteoblast build bone. A low pH, or acidosis, inhibits the bone-building activity of osteoblasts, contributing to the overall bone loss. At a high pH of 7.4 or above, osteoclast activity is suppressed.
Muscle Cells
Blood acidosis can lead to muscle loss or degradation. Skeletal and cardiac muscle cells are affected. A low pH depresses cardiac muscle cell contractions. Smooth muscle cells are also impacted by acidosis. For example, vascular smooth muscle cells contract with increases in extracellular pH and relax with decreases in pH. A rise in extracellular pH increases the influx of calcium into vascular smooth muscle cells, while a decrease in pH inhibits calcium entry into cells.
