Pediatric and Neonatal Resuscitation
Published (updated: ).
Pediatric and neonatal resuscitation involves algorithmic approaches to achieving the return of spontaneous circulation (ROSC) that is similar to adult cardiorespiratory resuscitation but requires special considerations in terms of differential diagnoses, medication dosing, procedures, and continuation of care that makes this subject dissimilar.
Anatomy and Physiology
Neonatal and pediatric patients differ from adults, both anatomically and physiologically, in many aspects that can affect resuscitation. A child’s jaw is shorter than in adults, reducing the available room to maneuver. The hypopharynx is shorter and narrower, with a more anterior location of the vocal cords. This further complicates an initial approach already affected by a proportionally larger head to body size. This limited view is further reduced with the presence of large tonsils and adenoids.
Prematurity, congenital complications, and maternal factors can add to the difficulty in resuscitating neonates. In a crashing patient, familiar causes of cardiac arrest should be considered and treated if present. This includes trauma, tamponade, pneumothorax, and shock due to blood loss or infection. In children, cardiomyopathies and myocarditis should also be included in a differential as their preceding symptomatology may have gone unnoticed. Metabolic derangements secondary to internal disease processes, such as sepsis or toxic ingestion, may result in arrhythmias. In these instances, correction of the underlying cause should be addressed in addition to CPR.
Indications
Resuscitation should always begin immediately if a patient becomes cyanotic, asystolic, or is in respiratory arrest but can be initiated if the patient is ill-appearing and their heart rate is <60 bpm. If a patient has a pulse, rescue breaths are indicated if impending airway failure is suspected. Up to 10% of newborns require medical assistance to begin breathing when born. Approximately 1% will require intensive support. While support should be initiated immediately, all neonates will initially be hypoxic. SpO2 after birth should be around 60% and then rise approximately 10% every 2 minutes subsequently.
Contraindications
Although there is an instinctual drive to do everything in one’s power to save the life of a child, in neonates and infants that suffer from congenital malformation or disease which significantly lower life expectancy or quality of life, it is considered reasonable to withhold resuscitative measures, this includes significant prematurity.
In children, there are no recommended time limits to discuss an end to resuscitation. However, in neonates, studies have not evaluated significant survival rates beyond 20 minutes, and, therefore, resuscitative efforts can be terminated at any point past this timeframe. As in any medical case involving end-of-life decisions, family, and appropriate staff, such as palliative care and specialists, should be included to assist in determining the right course for the patient’s care ethically and in the eyes of the family.
Equipment
If a patient requires defibrillation or cardioversion, biphasic attenuated defibrillators are preferred over adult AEDs which are non-attenuated. While this is preferred, adult AEDs may be used if a pediatric AED is unavailable. 2 to 4 J/Kg can be used for defibrillation, with the recommendation being to start at lower energy doses.
Technique
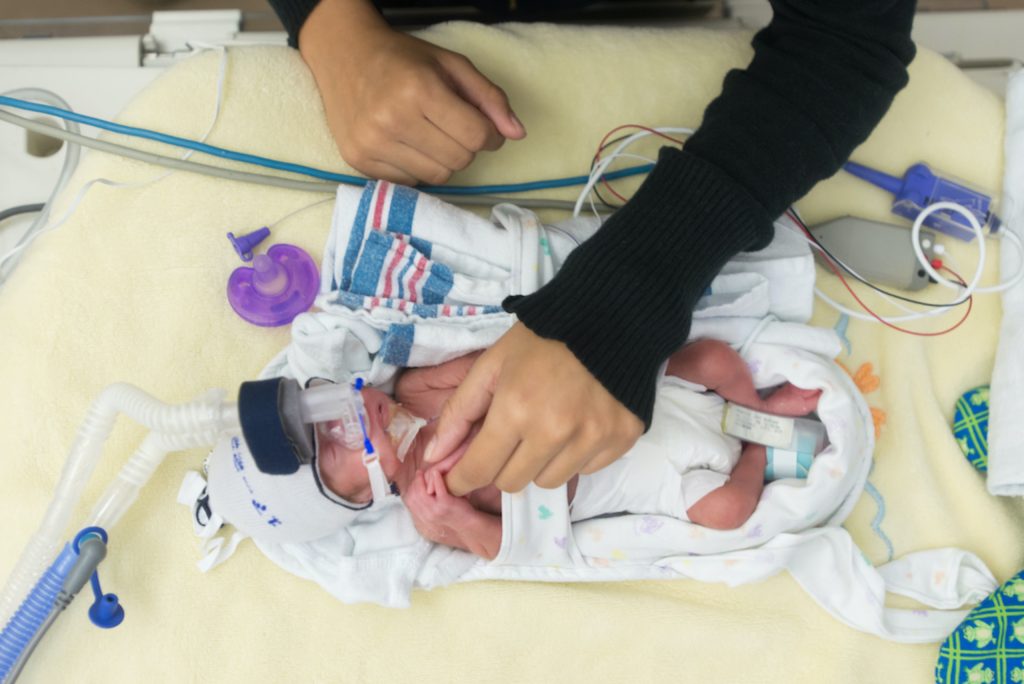
When born, drying and warming the baby is vital for the avoidance of hypothermia. While skin-on-skin contact between mother and baby is considered the standard form of initial warming, aids in feeding and temperature control, further warming may be needed. If additional temperature-controlling methods are needed, then wraps, radiant warmers, warm rooms, and heated, humidified air can all be utilized. If the birth occurs in a resource-poor environment, a plastic bag placed around the newborn’s body to the neck (leaving the head exposed) and then wrapping the baby is an acceptable method of warming. In neonates, to reduce the risk of decompensation, cord clamping should occur no sooner than 30 seconds after birth and can even be delayed up to one minute. This decreases the need for cardiovascular support and is a simple preventative step. However, if a neonate is in significant distress immediately after birth, cord clamping should not delay resuscitation.
While cord milking has occasionally been advocated for in the past, newer studies have shown that it is not beneficial. In preterm neonates, cord milking can actually cause significant harm and increase mortality. Studies are not yet conclusive on neonatal susceptibility to COVID-19. This includes instances where the patient is born to a COVID-19 positive mother. Hence, recommendations vary greatly between countries on subjects such as skin-to-skin contact and cord clamping. These changes differ from what is suggested by the AHA guidelines outlined in this article and do not have sufficient evidence to support widespread adoption at this time.
If a patient’s O2 saturation does not adequately increase, O2 delivery via nasal cannula is recommended as a first-line. Suction is not empirically recommended. O2 delivery can be started at 30% and titrated to a SpO2 saturation above 94%. If a patient does not quickly improve, the method of addressing the hypoxia must change, and differential diagnoses including cardiac malformations, congenital airway malformation, and metabolic abnormalities should be considered. In these patients, positive pressure ventilation (PPV) should be initiated at 40-60 breaths per minute, and intubation may be required.
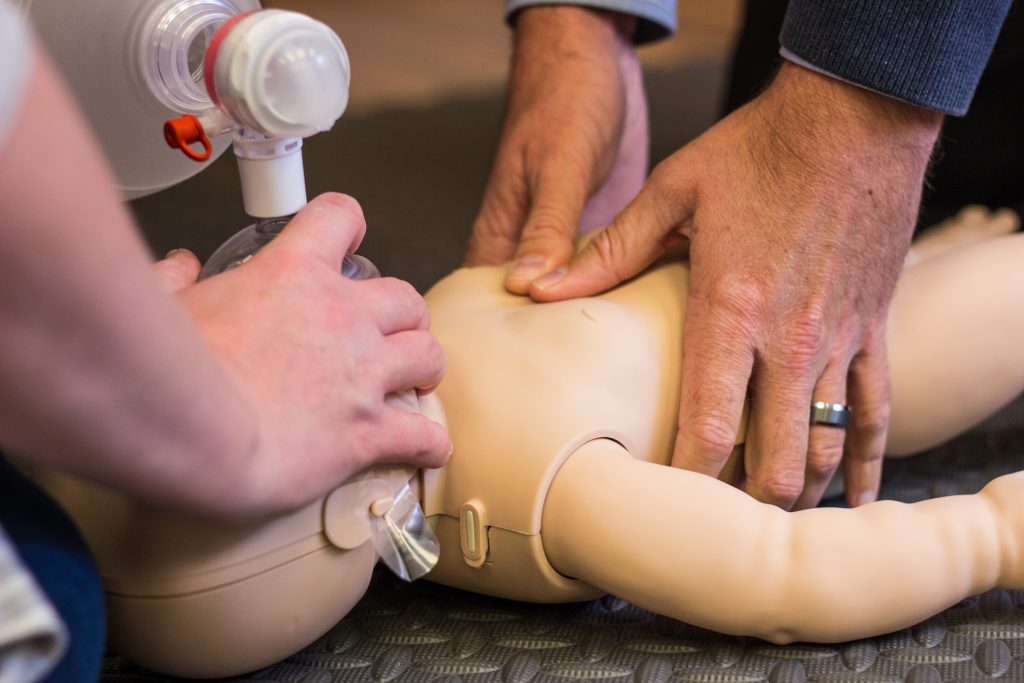
CPR and airway management should not be withheld if a patient meets the criteria mentioned in the indication section. The two-finger or two-thumb methods of chest compressions are both acceptable means of performing CPR. The compression-to-breath ratio is 30 to 2 for a single provider and 15 to 2 if assistance can be provided. Approximately 20 to 30 rescue breaths should be given per minute for adequate ventilation accompanied by 100 to 120 chest compressions.
Fluid status is, therefore, an important consideration in pediatric management. There is not sufficient clinical evidence to support choosing balanced versus unbalanced crystalloid fluids. These can be administered at 10 to 20 mL/Kg, with a maximum of 60 mL/Kg, with a vasopressor added as necessary for refractory cases. Both at a starting dose of 0.05 mcg/kg/min, norepinephrine and epinephrine are considered first-line agents for cold and warm shock, respectively. Dopamine is considered the second line in both cases, with a starting rate of 10 mcg/kg/min.
Medication dosing for children is almost always weight-based. The use of length-based tapes can assist in rapid calculations if actual weight cannot be obtained. Route of administration does have some more variability than adults. In neonates under 14 days old, a 5 F IV into the umbilical vein is a first-line and reliable option.
Suffocation from household objects and foreign body ingestion are common etiologies of decompensation in pediatric patients. In a choking patient, coughing and/or wheezing is a sign that the airway is likely still patent, whereas cyanosis or a lack of sound indicates no air passage. If a foreign object is visible during the airway examination and can be easily extricated, the object can be removed. If no object is visible or able to be extricated easily, utilize back blows, abdominal thrusts, or, if necessary, chest compressions to aid the patient in dislodging the obstructing body. If available, ENT, pulmonology, gastroenterology, or, possibly, surgery should be contacted to evacuate the object. Do not perform blind finger sweeps as this may further impact the offending object. If it is not visible or extractable, imaging studies and specialist care may be required. Common resuscitative medications such as sodium bicarbonate and calcium, unless indicated by the cause of decompensation, should not be given empirically or without reason, as they have been associated with a higher mortality rate.
Complications
Although stabilization can be performed at most facilities, definitive treatment or long-term care is usually restricted to larger, or child-specific, centers. Beginning preparations by contacting the necessary physicians should be completed early in resuscitation. Depending on availability, ECMO/ECPR should be considered early during the code for in-hospital arrests. This has not been verified in out-of-hospital arrests, however, if clinical judgment or discussion with a specialist suggests a possible benefit, and procedural criteria are met, this option cannot be excluded from your algorithm.
While age is usually correlated with improved outcomes post-resuscitation, post-arrest brain injury remains a cause for significant morbidity and mortality even in young populations. Therefore, EEGs and seizure prophylaxis are recommended for patients at risk after ROSC. Patients that have unknown causes of death should undergo an autopsy with a possible subsequent genetic analysis. Although not required, this should be emphasized to the family as being important in order to be proactive in preventing further family deaths if the cause is determined to be genetic.
