Hypovolemic Shock Treatment
Published .
Introduction
At its most basic definition, the term “shock” means that there is a lack of adequate tissue oxygenation throughout the body. Typically, this lack of oxygenation is caused by either a lack of circulating blood volume, a decrease in cardiac function, a decrease in systemic vascular resistance, or some other means by which the body cannot circulate blood flow to vital organs. Typically associated with this is a sudden drop in blood pressure by which the body cannot adequately perfuse vital organs. When trying to resuscitate a patient in shock, it is important to keep in mind the etiology of the patient’s shock, as this will sometimes drastically alter the process of proper resuscitation.
The most important type of shock that is encountered in resuscitation is a hemorrhagic shock. Closely related to this would be a more broad category of hypovolemic shock. Aside from acute hemorrhage, cardiogenic shock describes a cause of shock in which the cardiac output is strictly the component by which the body cannot perfuse the rest of the body. Neurogenic and septic shock deal with a decrease in systemic vascular resistance which prevents the body from directing blood to vital organs due to the decreased pressure. The shock from adrenal insufficiency is another cause that incorporates factors of cardiac output (CO), systemic vascular resistance (SVR), and volume.
Roughly 21 million blood products are transfused every year in the United States. Daily, almost 36000 packed RBCs, 7000 platelets, and 10000 FFP units are transfused in the United States. However, the vast majority of these transfusions are not due to hemorrhagic shock, but to prepare a patient for elective surgery (55%), or for patients with some form of chronic anemia (30%). Only 15% of all transfusions in the United States are initiated due to an emergent need for blood products or trauma. Despite the relative infrequency of hemorrhagic shock, it is important to understand the current guidelines regarding appropriate fluid resuscitation in this situation.
Indications
When defining hemorrhagic shock, it is categorized based on the severity of impact on systemic circulation. The corresponding class of shock then determines appropriate intervention.
Class 1 hemorrhagic shock is defined by a blood loss of up to 750mL (or up to 15%) of total blood volume. At this stage, the remainder of the patient’s perfusion parameters is still within normal limits. The patient’s heart rate will typically remain under 100 bpm and their blood pressure and pulse pressure will remain stable if not slightly increased due to anxiety. The respiratory rate is stable at 14-20 breaths/min, and their urine output remains at greater than 30 mL/hr.
In Class 2 shock, the patient has lost 750 mL to 1500 mL of blood (between 15% and 30% of total blood volume) and is beginning to become symptomatic. They may start to appear more pale or diaphoretic, with mild tachycardia (100-120 bpm), the respiratory rate may increase slightly (20-30 bpm), and their urine output may drop slightly (20-30 mL/hr). It is important to note that even outside the realm of shock management, urine output remains the single most important indicator for monitoring fluid status in a patient. Additionally, blood pressure cannot be adequately relied upon to detect the beginning of shock, as the body’s compensatory mechanisms will keep blood pressure typically within normal limits until up to 30% of total blood volume has already been lost. Finally, the Class 2 shock patient may demonstrate a slight decrease in pulse pressure, which may be the first sign of the body failing to compensate for the sudden blood loss.
In Class 3 hemorrhagic shock, the patient has lost 1500-2000 mL of blood (30-40% total blood volume), they will be clearly symptomatic, confused and will be tachycardic (120-140 bpm), tachypneic (30-40 breaths/min), with blood pressure and pulse pressure decreased causing a drop in renal perfusion (urine output decreased to 5-15 mL/hr). Class 4 shock is the most severe case with acute blood loss of over 2000 mL (or over 40% total blood volume). The patient’s heart rate will be tachycardic, over 140 bpm, with nonpalpable or thready peripheral pulses. Their respiratory rate will have increased to over 35 breaths/min, and their blood pressure and pulse pressure will subsequently be severely decreased. Urine output will be negligible, and symptomatically they will be more lethargic with a likely altered mental status.
Equipment
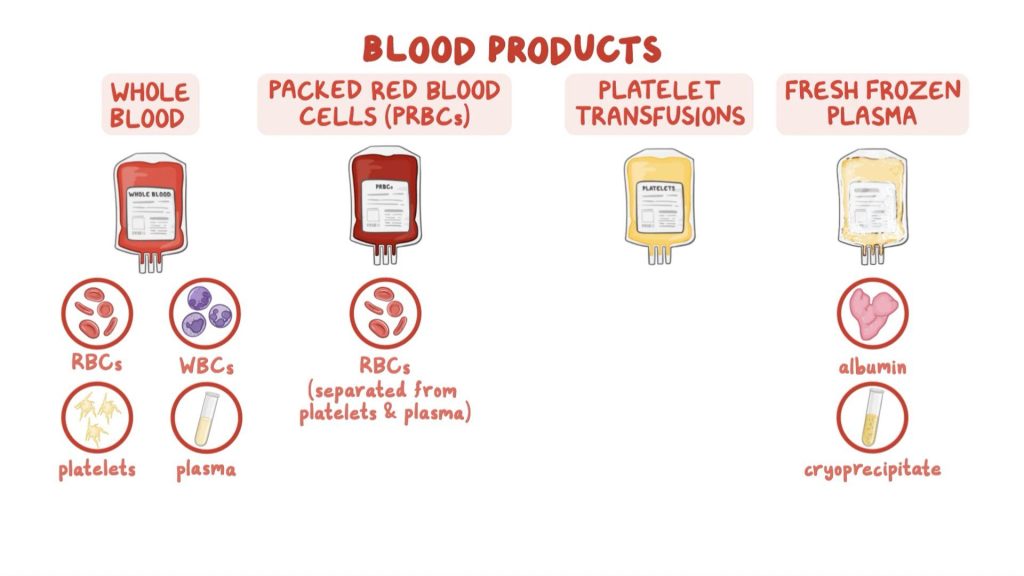
Packed red blood cells are provided in units of roughly 350 cc, and are more concentrated than whole blood with a hematocrit of 65-75%. The plasma and platelets are removed via centrifuge, and the remaining packed red blood cells are stored in a saline-based preservative such as citrate phosphate dextrose adenine (CPDA-1) for increased shelf life. Packed RBCs (pRBCs) can be stored for up to 35 days at 2-4 degrees Celsius. One unit of pRBCs is thought to raise a patient’s hemoglobin level by 1g/dL. These products must be typed and matched for ABO and Rh compatibility with patient recipients.
Fresh frozen plasma is given in units of 200-250 cc each and contains all coagulation factors, with no red blood cells or platelets. For FFP to be therapeutic, it is required to be given at 10-20 cc/kg body weight, which would theoretically increase the body’s clotting factor levels by 20-30%. For an increased shelf life of up to 2 years, they are frozen within 8 hours of collection and stored at -40 to -50 degrees Celsius. They are then thawed and must be used immediately as their thawed shelf life is only 5 days before they begin to degrade. Frozen plasma (FP), which is less commonly used, and typically frozen within 24 hours of collection (FP24), has slightly reduced levels of factor 5 and 8 as compared to FFP. FFP is particularly useful for certain coagulopathies or in isolated clotting factor deficiencies. There is some speculation as to the benefit of FFP in patients with multiple clotting factor deficiencies or coumadin coagulopathy, but its standard use in the hemorrhagic shock patient remains valid.
Platelets are given in high concentration “6 packs” of platelets with one “6 pack” being equal to one apheresis Unit. 1 Unit is typically 250 cc, is stored concentrated in a small volume of plasma, and only has a shelf life of 5 days at 20-24 degrees Celsius. Unlike pRBCs, platelets lose what little shelf life they have when they are frozen, and so must remain fresh from collection to administration. One Unit of platelets is thought to increase the body’s platelet count by 30,000-60,000 platelets/uL. Roughly 20% of patients can develop antiplatelet antibodies after 10-20 transfusions.
Technique
Initial hemorrhagic shock resuscitation begins with the administration of IV fluids, followed by transfusion of blood products at a 1:1:1 ratio. The initial IV fluids should be a 2 L bolus of 0.9% normal saline or two 20 mL/kg boluses by patient weight. Typically, patients in Class 1 or 2 can be treated initially with a trial bolus of crystalloids, but patients in Class 3 or 4 should be getting blood products immediately with the first bolus of crystalloids. The amount of blood transfused depends on a variety of factors, but is specifically centered around the concept of “permissive hypotension”. Permissive hypotension is the idea that a patient in active hemorrhagic shock should be transfused just enough blood products to retain a systolic blood pressure above 70 mmHg. Then, after hemorrhage is controlled, the patient can be transfused to retain a systolic blood pressure above 90 mmHg. As a rule of thumb, one can expect roughly a loss of 1 L blood with a femur fracture, and at least 1 L blood loss with a pelvic fracture. Other long bone fractures such as the humerus, tibia, or fibula can also account for as much as 500 ccs each of blood loss. As such, a patient with bilateral femur fractures or a pelvic fracture can already be assumed to be approaching stage 3 or IV of hemorrhagic shock. As the saying goes in accounting for blood loss in hemorrhagic shock, “blood on the floor, plus four more”. This phrase meaning basically that a life-threatening amount of blood can be lost as active hemorrhage outside the body, in the thigh compartments of bilateral femur fractures, the pelvis, abdomen, or chest. It should also be noted that no number of transfusions should be used as a substitute for definitive control of an active bleed.
Clinical Significance
Shock resuscitation remains clinically significant simply due to the life-threatening nature of blood loss. Due to the body’s ability to bleed a significant amount into either the chest, abdomen, pelvis, or thighs, the first signs of hemorrhagic shock may not always be so easily noticed. In addition, many cases of shock resuscitation are not prompted by traumatic events, but by other forms of blood loss. Whether it be due to a GI bleed, or hemorrhage due to coagulopathy, physicians must be mindful of changes in vital signs should they occur in order to recognize shock. Furthermore, the patients’ general appearance may be helpful in determining the diagnosis. If the patient appears diaphoretic and visibly uncomfortable along with having vital signs suggestive of early stages of hemorrhagic shock, clinical suspicion for shock should be high.
A common sign of impending shock can be a decrease in pulse pressure, an increased heart rate, or a slight increase in breathing. Most important of all, the clinical evidence of decreased urine output can indicate impending shock as kidneys become slightly hypoperfused. A decrease of urine output below 30 ccs/hr, or more exactly less than 0.5 ccs/kg/hr, suggests renal hypoperfusion and could be the first sign of stage 1 shock. Of course, there are other reasons which could be causing renal hypoperfusion. It is vital to keep in mind the patient’s full history and physical exam, as these may suggest other etiologies besides blood loss. For example, if a patient has been taking a new beta-blocker, they may be having decreased blood pressure and renal hypoperfusion unrelated to blood loss. The physical exam must be used to assess the chest, abdomen, pelvis, and thighs to ensure there is no evidence of blood loss present. These signs could be as apparent as decreased breath sounds due to blood in the pleural space, or increased abdominal distention or pain due to blood in the abdomen. Patients with some level of coagulopathy may also have hematomas that may develop along with the psoas, which may be difficult to diagnose on exam along. Any new pain in lower extremity flexion/extension should be the reason for concern.
