Intravenous Fluids
Published (updated: ).

Indications
Crystalloid fluids are a subset of intravenous solutions that are frequently used in the clinical setting. Crystalloid fluids are the first choice for fluid resuscitation in the presence of hypovolemia, hemorrhage, sepsis, and dehydration. Other clinical applications include acting as a solution for intravenous medication delivery, delivering maintenance fluid in patients with limited or no enteral nutrition, blood pressure management, and increasing diuresis to avoid nephrotoxic drug or toxin-mediated end-organ damage.
While normal saline (0.9% NaCl solution) is the most frequently used crystalloid fluid, many other formulations can provide improved clinical outcomes in specific patient populations.
Other commercially available crystalloid fluids include:
- Lactated Ringer’s/Hartman’s solution (lactate buffered solution)
- Acetate buffered solution
- Acetate and lactate buffered solution
- Acetate and gluconate buffered solution
- 0.45% NaCl (hypotonic solution)
- 3% NaCl (hypertonic solution)
- 5% Dextrose in water
- 10% Dextrose in water
Mechanism of Action
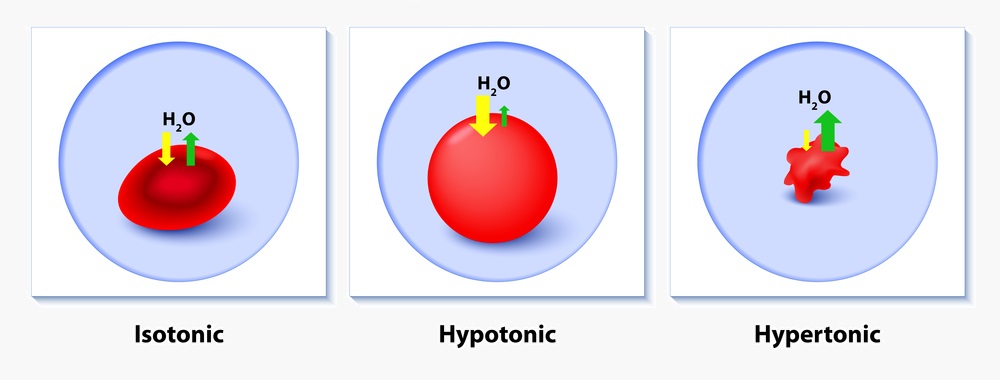
A crystalloid fluid is an aqueous solution of mineral salts and other small, water-soluble molecules. Most commercially available crystalloid solutions are isotonic to human plasma. These fluids approximate concentrations of various solutes found in plasma and do not exert an osmotic effect in vivo. Crystalloid fluids function to expand intravascular volume without disturbing ion concentration or causing significant fluid shifts between intracellular, intravascular, and interstitial spaces.
Hypertonic solutions such as 3% saline solutions contain higher concentrations of solutes than those found in human serum. Because of this discrepancy in concentration, these fluids are osmotically active and will cause fluid shifts. Their primary indication is for emergent replacement of serum solutes, such as in hyponatremia with neurologic symptoms.
Buffered solutions contain molecules that metabolize in vivo to bicarbonate. These solutions were designed to sustain a normal physiologic plasma pH. The three commonly used molecules are lactate, acetate, and gluconate. Lactate and gluconate are hepatically metabolized to bicarbonate, while acetate is predominantly metabolized peripherally by skeletal muscle.
Administration
Crystalloid fluids are administered parenterally via an intravenous infusion. Infusion rates depend on the clinical presentation and indication for administration.
Fluid Resuscitation
In an acute setting, the clinical situation may indicate a rapid infusion of crystalloid fluids. Such cases may require a pressure apparatus to the bag of fluid to achieve a higher infusion rate. In this clinical setting, larger bore intravenous cannulas should help ensure the safe administration of high fluid volumes. Fluids should be administered, preferably via large-bore peripheral lines (18-gauge or larger) or through central access, which may also be used to deliver blood products if required.
Maintenance Fluids
The fluid requirements of patients were determined to be related to a patient’s caloric demand by Drs. Holliday and Segar in 1957. Since this time, their initial formula has been modified to provide clinicians with guidelines for administering maintenance crystalloid fluids. The mass-based formula uses what is known as the “4-2-1” rule:
- 0-10 kg: +4 mL/kg/hr
- 10-20 kg: +2 mL/kg/hr
- >20 kg: +1 mL/kg/hr
Example: 100 kg patient: 20 kg (40 + 20 mL/hr) + 80 kg (80 mL/hr) = 140 mL/hr
Additional formulas for fluid administration have been developed for specific clinical scenarios (e.g., the Parkland Formula for fluid maintenance in burn patients).
Adverse Effects
Volume expansion with crystalloid fluids may cause iatrogenic fluid overload. The risk of this complication becomes particularly elevated in patients with impaired kidney function (acute kidney injury, chronic kidney disease, etc.), and these patients should, therefore, receive treatment with judicious use of intravenous fluids. Patients with congestive heart failure are at elevated risk for serious adverse effects of crystalloid fluid administration. Fluid overload can cause life-threatening pulmonary edema and the worsening of diastolic or systolic heart failure, leading to end-organ damage or even death. It is vital for the clinician to monitor these patients carefully and administer the minimum required volume to maintain volume homeostasis.
Normal saline (0.9% Saline)
Normal saline has a higher concentration of chloride ions (154 mmol/L) than in human serum (98 to 106 mmol/L). With the administration of large volumes of normal saline, hyperchloremia occurs. While there is still some debate on the exact mechanism of this pH disturbance, the thinking is that the increase in chloride concentration adjusts the substantial ion difference in plasma, resulting in more free water in the intravascular space. As a result, the hydrogen ion concentration in the serum would increase to maintain electrochemical neutrality. Excessive renal bicarbonate excretion can occur, resulting in metabolic acidosis. The dilution of serum bicarbonate through non-buffered crystalloids (e.g., normal saline) may also contribute to acidosis. Besides metabolic acidosis, clinical research has shown that high volumes of normal saline can cause hyperchloremia-induced renal afferent arteriole constriction, which can cause a decrease in the glomerular filtration rate.
Acetate buffered crystalloid solutions have been the subject of much debate in the medical literature. Studies performed on dogs have shown that even small volumes of acetate-containing crystalloids can significantly increase the serum concentration of acetate to 10 to 40 times the physiologic level. Some suggest that acetate may potentiate hemodynamic instability by decreasing both myocardial contractility and blood pressure.
Unlike acetate buffered solutions, lactated crystalloid fluids have the potential to induce hyperglycemia. Lactate is a metabolically active compound that is utilized during gluconeogenesis to produce glucose. Hence, excessive administration of lactated crystalloids may be of concern in patients with diabetes.
Contraindications
- Patients who are fluid-overloaded should not receive crystalloid fluids. Special care is prudent when administering fluids to patients with congestive heart failure or those with significant renal impairment (e.g., CKD-V dialysis-dependent patients).
- Hypertonic saline is contraindicated in all clinical settings except in patients with severe hyponatremia and neurologic sequelae. Rapid correction of hyponatremia may cause central pontine myelinolysis, a devastating neurologic condition.
- Hypotonic solutions are also contraindicated in patients with or at risk of developing cerebral edema.
- Crystalloids containing potassium (Lactate Ringer’s solution, Hartman’s solution, etc.) are relatively contraindicated in patients with hyperkalemia since these may exacerbate their condition, which in turn can lead to ventricular dysrhythmias.
- Clinicians should avoid using crystalloids containing dextrose (D5%W, D10%W, D5% 0.45% NS, etc.) in patients with hyperglycemia.
- Ringer’s lactate solution contains calcium ions. Calcium can induce coagulation of the blood products in the IV tubing and therefore inhibit their effective delivery. In patients who require a blood transfusion, blood products should utilize a separate IV setup.
Monitoring
Patients should undergo assessment for signs and symptoms of dehydration and fluid overload. Indications that a patient may receive inadequate volume include elevated lactate and creatinine concentrations in the absence of an alternate cause. The urine output also requires monitoring. An ideal urine output target of 0.5 mL/kg/hr indicates adequate hydration but may not be useful to assess volume status in patients with renal impairment.
To monitor for fluid overload, patients at high risk of developing this complication should receive frequent re-evaluation. Providers should assess for new or worsening crackles. These sounds may indicate pulmonary edema secondary to volume overload. Additionally, any new or worsening peripheral edema in the extremities is also a potential indication of excessive crystalloid fluid administration.
In patients receiving hypertonic saline for severe hyponatremia with neurologic sequelae, frequent neurologic checks are necessary to assess clinical improvement. Such monitoring can also help identify worsening neurologic function as a potential indicator of cerebral edema or central pontine myelinolysis.
Patients receiving large volumes of crystalloid fluids should be monitored for electrolyte imbalances caused by crystalloid fluid administration.
