Pharmacokinetics
Published (updated: ).
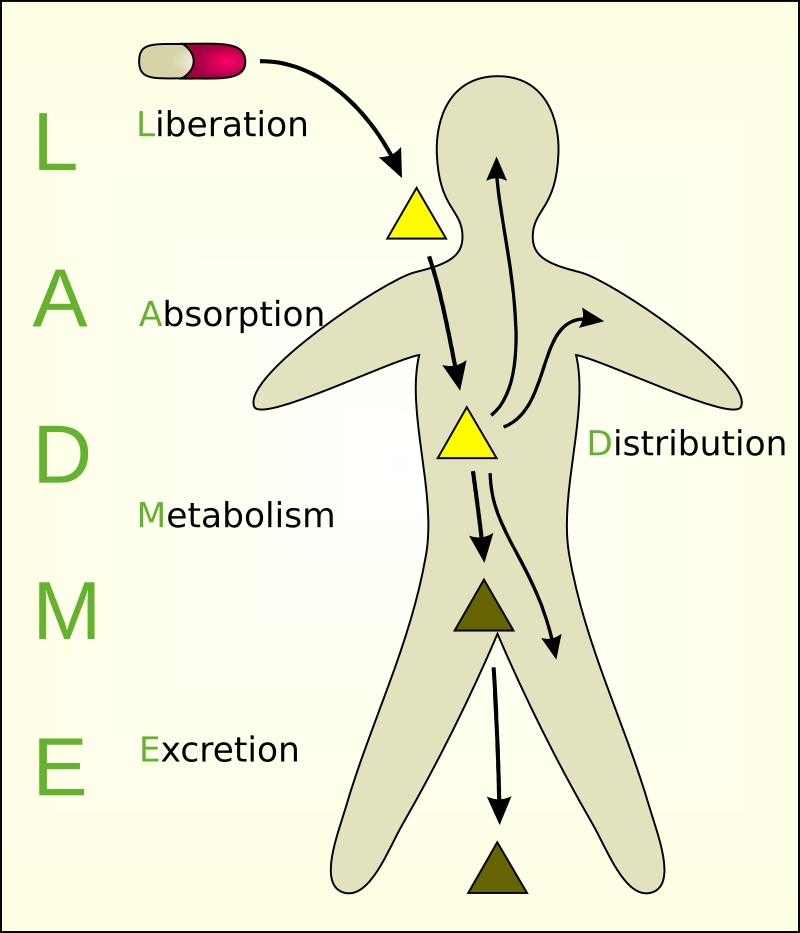
Pharmacokinetics (PK) is the study of how the body interacts with administered substances for the entire duration of exposure (medications for the sake of this article). This is closely related to but distinctly different from pharmacodynamics, which examines the drug’s effect on the body more closely. The four main parameters generally examined by this field include absorption, distribution, metabolism, and excretion (ADME). Wielding an understanding of these processes allows practitioners the flexibility to prescribe and administer medications that will provide the greatest benefit at the lowest risk and allow them to make adjustments as necessary, given the varied physiology and lifestyles of patients.
Issues of Concern
Absorption
Absorption is the process that brings a drug from the administration, e.g., tablet, capsule, into the systemic circulation. Absorption affects the speed and concentration at which a drug may arrive at its desired location of effect, e.g., plasma. There are many possible methods of drug administration, including but not limited to oral, intravenous, intramuscular, intrathecal, subcutaneous, buccal, rectal, vaginal, ocular, otic, inhaled, nebulized, and transdermal. Each of these methods has its own absorption characteristics, advantages, and disadvantages.
The process of absorption also often includes liberation, or the process by which the drug is released from its pharmaceutical dosage form. This is especially important in the case of oral medications. For instance, an oral medication may be delayed in the throat or esophagus for hours after being taken, delaying the onset of effects or even causing mucosal damage. Once in the stomach, the low pH may begin to chemically react with these drugs before they even arrive in the systemic circulation.
Bioavailability
Bioavailability is the fraction of the originally administered drug that arrives in systemic circulation and depends on the properties of the substance and the mode of administration. It can be a direct reflection of medication absorption. For example, when administering medication intravenously, 100% of the drug arrives in circulation virtually instantly, giving this method a bioavailability of 100%. This makes intravenous administration the gold standard regarding bioavailability. This concept is especially important in orally administered medications.
Oral medications, once swallowed, must navigate the acidity of the stomach and be taken up by the digestive tract. The digestive enzymes begin the process of metabolism for oral medications, already diminishing the amount of drug arriving in circulation before being taken up. Once absorbed by gut transporters, the medications then often have to undergo “first-pass metabolism.” When oral medication is administered, it is often processed in large quantities by the liver, gut wall, or digestive enzymes, subsequently lowering the amount of medication that arrives in circulation; therefore, having a lower bioavailability.
These processes will be discussed in greater detail under metabolism. Other modes of administration may delay certain quantities of drugs to arrive in circulation at the same time (intramuscular, oral, transdermal), giving rise to the use of the area under the plasma concentration curve (AUC). The AUC is a method of calculating the drug bioavailability of substances with different dissemination characteristics, and this observes the plasma concentration over a given time. By calculating the integral of that curve, bioavailability can be expressed as a percentage of the 100% bioavailability of intravenous administration.
Distribution
Distribution describes how a substance is spread throughout the body. This varies based on the biochemical properties of the drug as well as the physiology of the individual taking that medication. In its simplest sense, the distribution may be influenced by two main factors: diffusion and convection. These factors may be influenced by the polarity, size, or binding abilities of the drug, the fluid status of the patient (hydration and protein concentrations), or the body habitus of the individual. The goal of the distribution is to achieve what is known as the effective drug concentration. This is the concentration of the drug at its designed receptor site. To be effective, a medication must reach its designated compartmental destination, described by the volume of distribution, and not be protein-bound in order to be active.
Metabolism
Metabolism is the processing of the drug by the body into subsequent compounds. This is often used to convert the drug into more water-soluble substances that will progress to renal clearance or, in the case of prodrug administration such as codeine, metabolism may be required to convert the drug into active metabolites. Different strategies of metabolism may occur in multiple areas throughout the body, such as the gastrointestinal tract, skin, plasma, kidneys, or lungs, but the majority of metabolism is through phase I (CYP450) and phase II (UGT) reactions in the liver. Phase I reactions generally transform substances into polar metabolites by oxidation allowing conjugation reactions of Phase II to take place. Most commonly, these processes inactivate the drug, convert it into a more hydrophilic metabolite, and allow it to be excreted in the urine or bile.
Excretion
Excretion is the process by which the drug is eliminated from the body. The kidneys most commonly conduct excretion, but for certain drugs, it may be via the lungs, skin, or gastrointestinal tract. In the kidneys, drugs may be cleared by passive filtration in the glomerulus or secretion in the tubules, complicated by reabsorption in some compounds.
Clearance
Clearance is an essential term when examining excretion. It is defined as the ratio of the elimination rate of a drug to the plasma drug concentration. This is influenced by the drug, blood flow, and organ status (usually kidneys) of the patient. In the perfect extraction organ, in which blood would completely be cleared of medication, the clearance would become limited by the overall blood flow through the organ. An understanding of clearance allows practitioners to calculate appropriate dosing rates of medications. Maintenance dosing ideally replaces the amount of drug that was eliminated since the previous administration. It is calculated by clearance times the desired plasma concentration divided by bioavailability.
Half-life (t)
The half-life is the amount of time for serum drug concentrations to decrease by 50%. Clearance is directly proportional to the volume of distribution and inversely to clearance. The half-life of medications often becomes altered from changes in the clearance parameters that come with disease or age.
